Purpose:
To relate the physical and chemical properties of water to a water pollution issue.
Summary:
In this exercise, students will measure the temperature and dissolved oxygen of a stream and discuss what this information can tell us about possible pollution problems.
Background:
- To relate the physical and chemical properties of water to a water pollution issue.
- In this exercise, students will measure the temperature and dissolved oxygen of a stream and discuss what this information can tell us about possible pollution problems.
- During this activity students will investigate two properties of water in a stream – the temperature and the concentration of dissolved oxygen in the water. Students will explore how natural influences, human activities and pollution may cause these parameters to change.
- Temperature and oxygen were chosen for this activity because they are easy to measure, the causes of change are both varied and easy to understand, and also the two properties are related to each other. Fish and other animals living in water can be harmed by high temperatures and low oxygen concentrations.
- As water gets warmer the “saturation concentration” for oxygen gets lower – in other words the warmer the water, the less oxygen it can hold. Therefore, when water temperatures increase, fish are often hit with a double whammy of low oxygen as well.
Materials:
Documents:
Stream side Science Manual
When Things Heat Up Student
Presentations:
None

High temperatures or low dissolved oxygen are not necessarily a sign of a pollution problem in the stream. Temperatures change throughout the seasons and will also vary from year to year.
During warm drought years as opposed to wet years, temperatures in most streams will be higher during the summer because of lower flows and warm- er air temperatures. Therefore, the first thing to consider is whether you’re just observing natural changes in a stream. Stream standards allow for occasional violations because of this natural variation. Dissolved oxygen will be lower if the temperature is higher, and vice versa.
Human activities can increase the temperature in a stream.
Humans can affect the temperature of rivers by discharging heated water. Industrial or energy plants often produce heated water as a byproduct. Also, when we modify the stream banks (riparian area) and reduce the amount of canopy cover, we can have a direct impact on stream conditions without ever dumping in a pollutant.
Example:
Discharge water from energy plants and from some other industrial plants may be considerably warmer than the stream it discharges to. This type of “thermal pollution” is considered a point source (it travels from a source to a stream through a pipe or ditch). Your students should consider any such sources in their community. Many other human activities affect water quality through indirect means. Urban development, agricultural areas and logging areas may all result in removal of riparian vegetation along a stream. When the shade from these plants disappears, the stream is exposed to more sunlight and heats up. Therefore, your problem may just be some “brush clearing” activities upstream of your site.
Human activities can have decreased oxygen in a stream.
Oxygen can only get into water from the surface (mixing with the atmosphere) or from oxygen produced by plants in the water. Oxygen in water is consumed by animal and plant respiration, during various chemical reactions, and during the decay of organic material. Humans can have a profound effect on how much decaying material is in a stream.
Grass clippings, runoff from feedlots, and debris from logged areas are just a few of the sources of material which will ultimately decay in the water and in doing so, use up oxygen. In a rapidly moving stream, the water usually mixes with the atmosphere enough to replace this oxygen. In a pooled up or very slow-moving stream, especially if it’s warm, oxygen can be used up very quickly.
NOTE: Dumping nutrients into water (e.g., from yard fertilizers), can stimulate plant growth in a stream or lake. When these plants die, you may also see a drop in oxygen.
The time of day when measuring oxygen Concentration in a stream makes a difference
We often forget that plants not only create oxygen, but also use it for their cellmetabolism. During the night, plants do not photosynthesize but still use oxygen. In streams that have become congested with an overabundance of living plants, oxygen may be very high during the day, but can be extremely low just before dawn because of plant uptake.
Temperature
What is Temperature?
Temperature is the measure of how much heat energy water contains. A stream’s temperature is affected by the season, the source of water, the geographic area of the stream, the shape of the channel and whether the stream is shaded.
Most aquatic organisms require a specific temperature range, and many of our sport fish require cold water.
Temperature must be measured in the field. The temperature will change if the water is collected and stored, and will not reflect the true value at the site.
Discussion Questions for Temperature:
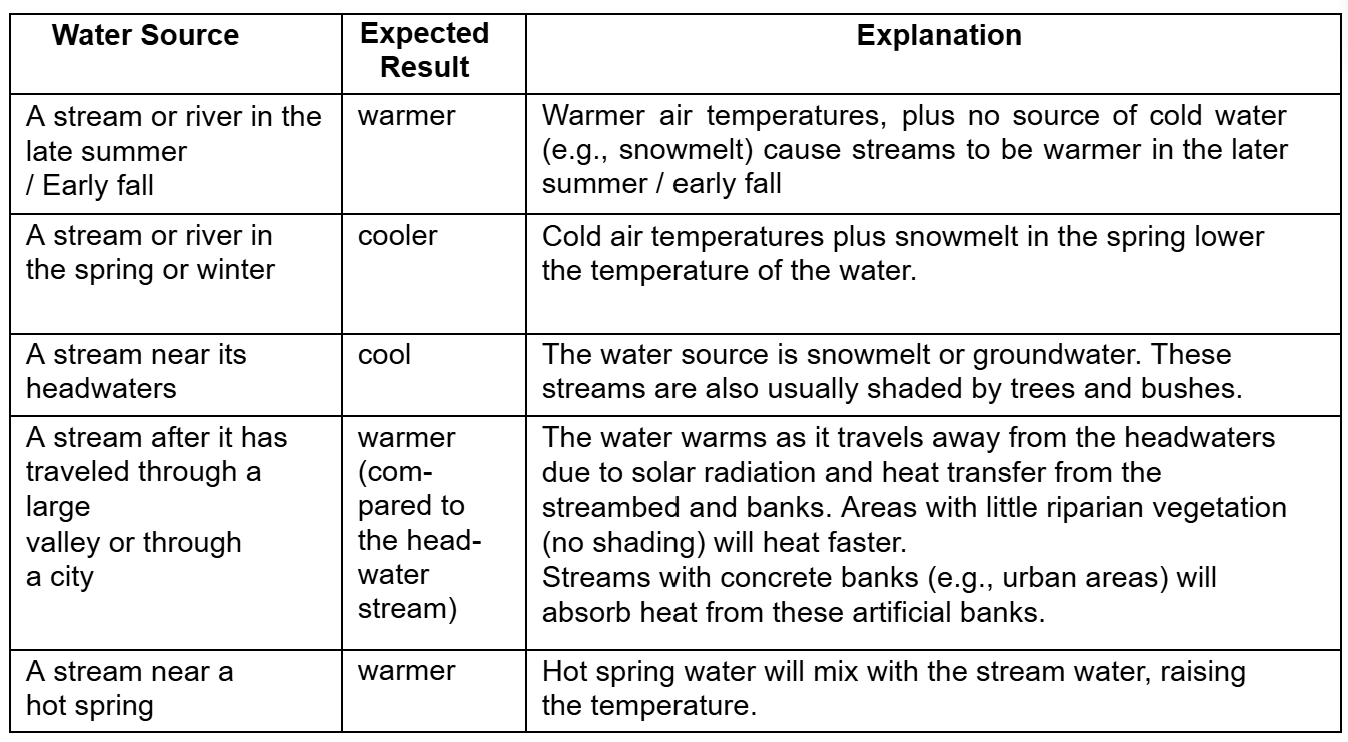
Groundwater entering a stream will affect its temperature.
Groundwater is usually colder than surface water and therefore it would probably cool the stream. Because the temperature of groundwater doesn’t fluctuate much throughout a year, a stream with a major groundwater component may show less seasonal variability than a stream fed entirely by surface runoff.
Different land uses affect stream temperature.
The major influences on temperature in a stream are exposure to the sun, and exposure to heated surfaces. Any activity that causes a stream to become shallower and wider (this can happen when too much sediment enters a stream) will cause the stream to heat more rapidly.
When trees along the banks are removed, the loss of shading can cause the stream to heat up.
Water that is diverted (such as for irrigation) and then returned to the stream usually heats up.
Finally, streams with small flows will heat faster than streams with lots of water, so removing water from a stream can cause an increase in temperature.
Suggested sources of water samples, with expected results and explanation:
Dissolved Oxygen
What is Dissolved Oxygen?
Dissolved oxygen (DO) is a measurement of the concentration of O2 molecules actually dissolved in water. This is the form of oxygen that fish and aquatic insects need.
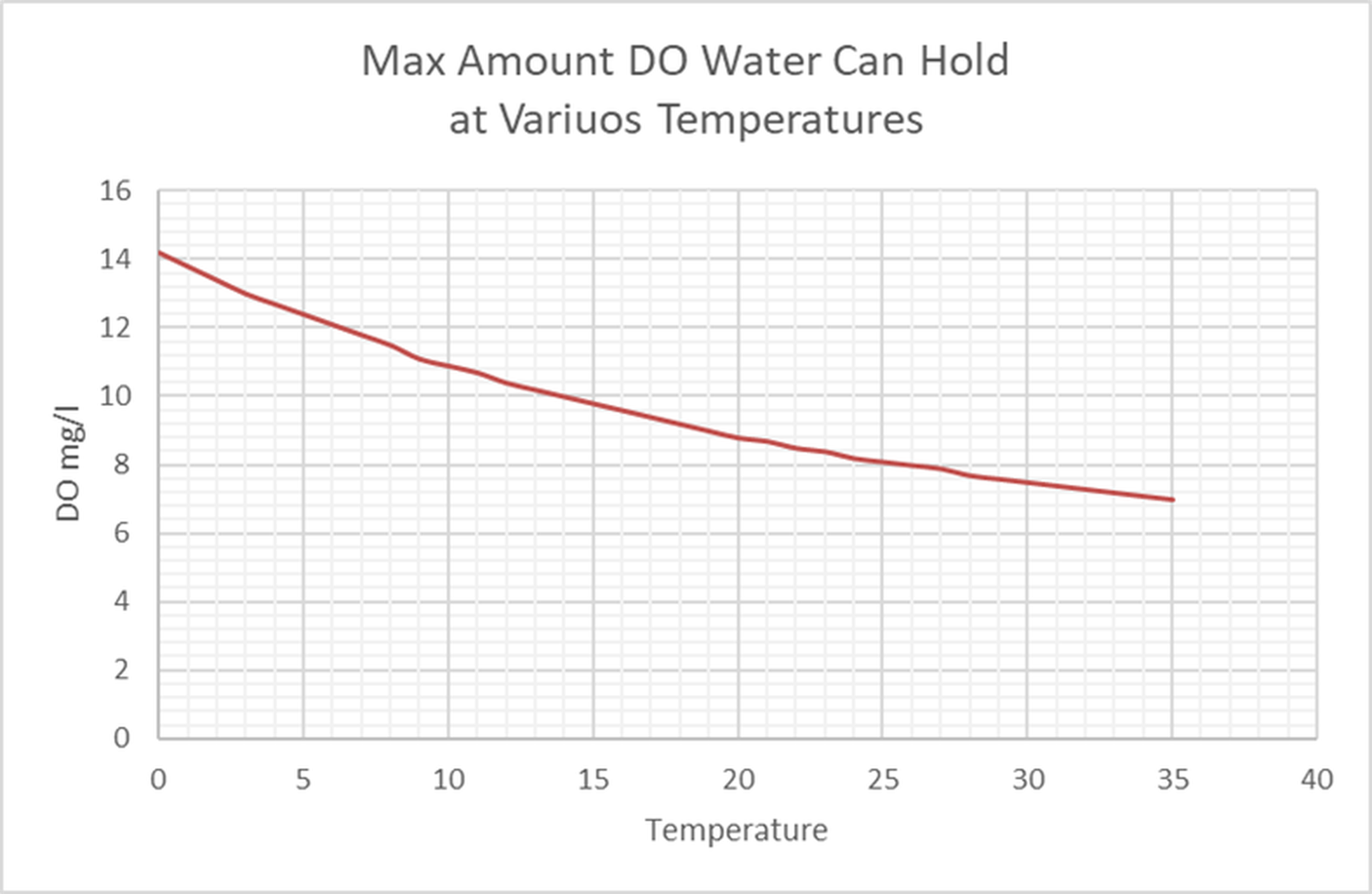
Oxygen is not very soluble in water. Usually, about 12 parts of oxygen can dissolve into a million parts of water. In very cold water however, concentrations can be as much as 14 parts per million (ppm) or mg/1. The maximum amount of oxygen that can dissolve in water is called its saturation concentration. The saturation concentration decreases as water temperature or elevation increase.
DO must be measured in the field. The DO will change if the water is collected and stored, and will not reflect the true value at the site.
Oxygen is dissolved into water by contact with the atmosphere, or from aquatic plants that produce oxygen during photosynthesis. Therefore, oxygen will be higher in turbulent stream water (lots of mixing with the atmosphere) or in water with lots of plants (but only during the day, when photosynthesis can occur).
The respiration of animals and plants uses oxygen. Bacterial decomposition of dead organic materials can be a major factor, and may cause the dissolved oxygen to be completely consumed in deep pools or lakes. Some chemical reactions (oxidation reactions) also require and consume oxygen.
The decomposition of organic materials such as these may use all the available oxygen in the water. Secondary treatment by municipal treatment plants removes the organic material from the water for just this purpose. Before municipal wastewater was treated properly, many rivers and streams had fish kills and dead zones caused by low oxygen as this waste was decomposed.
Effect of Temperature on Dissolved Oxygen Concentrations
The data below show the maximum amount of dissolved oxygen the water can hold at different temperatures. This is called the “saturation concentration” of oxygen.
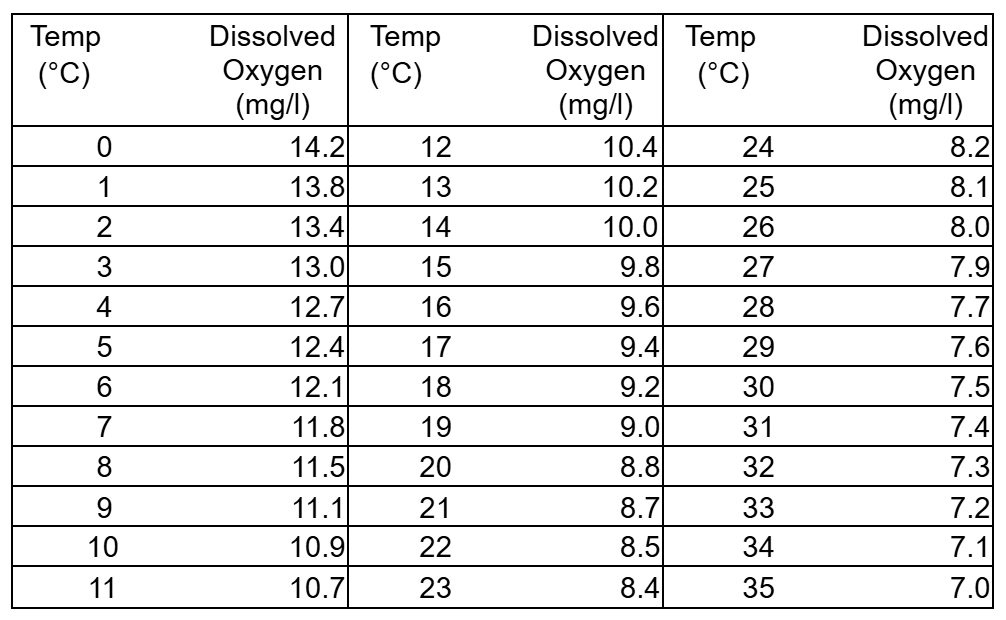
Create a graph showing the “saturation concentrations” of water as temperature changes.
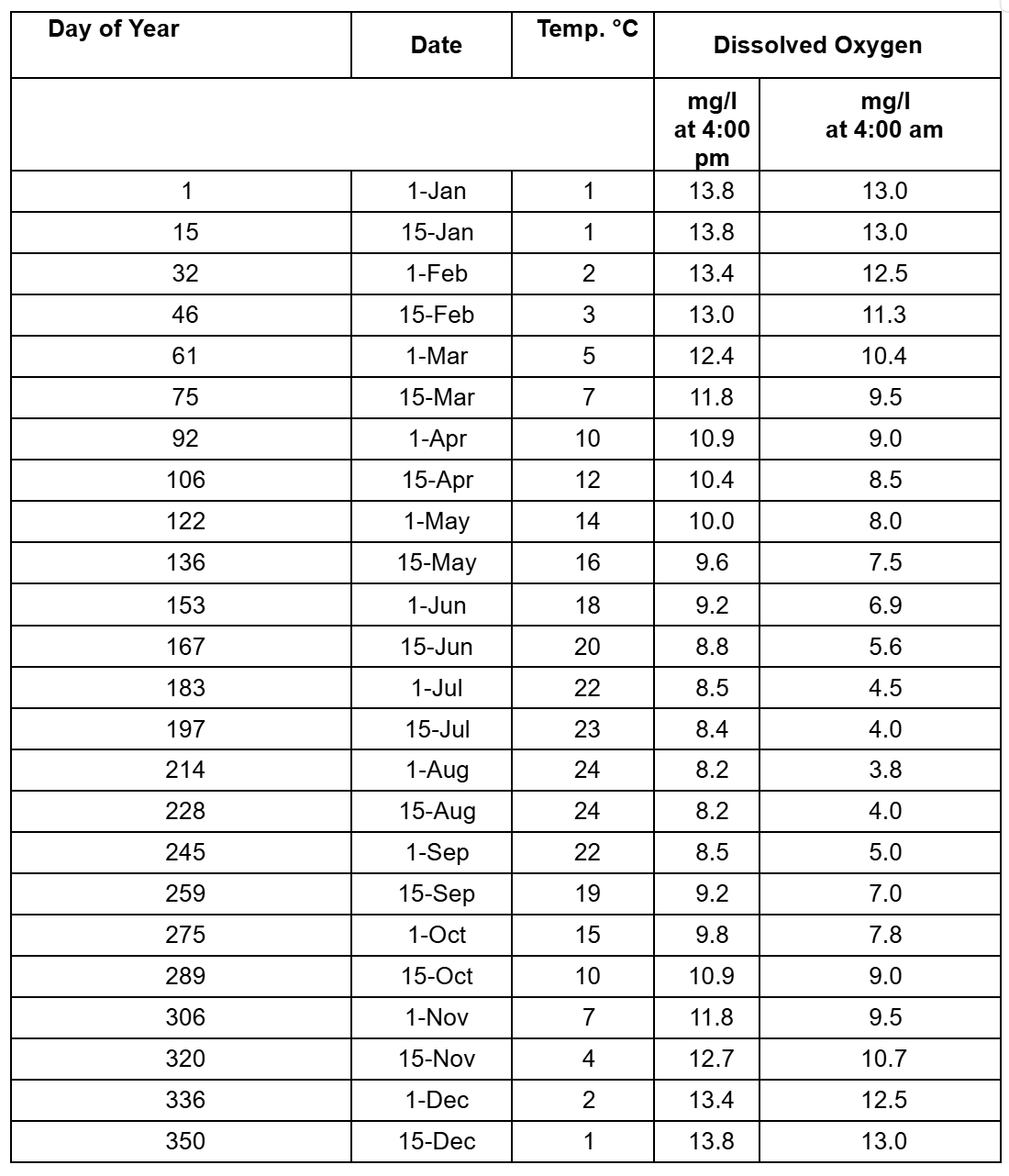
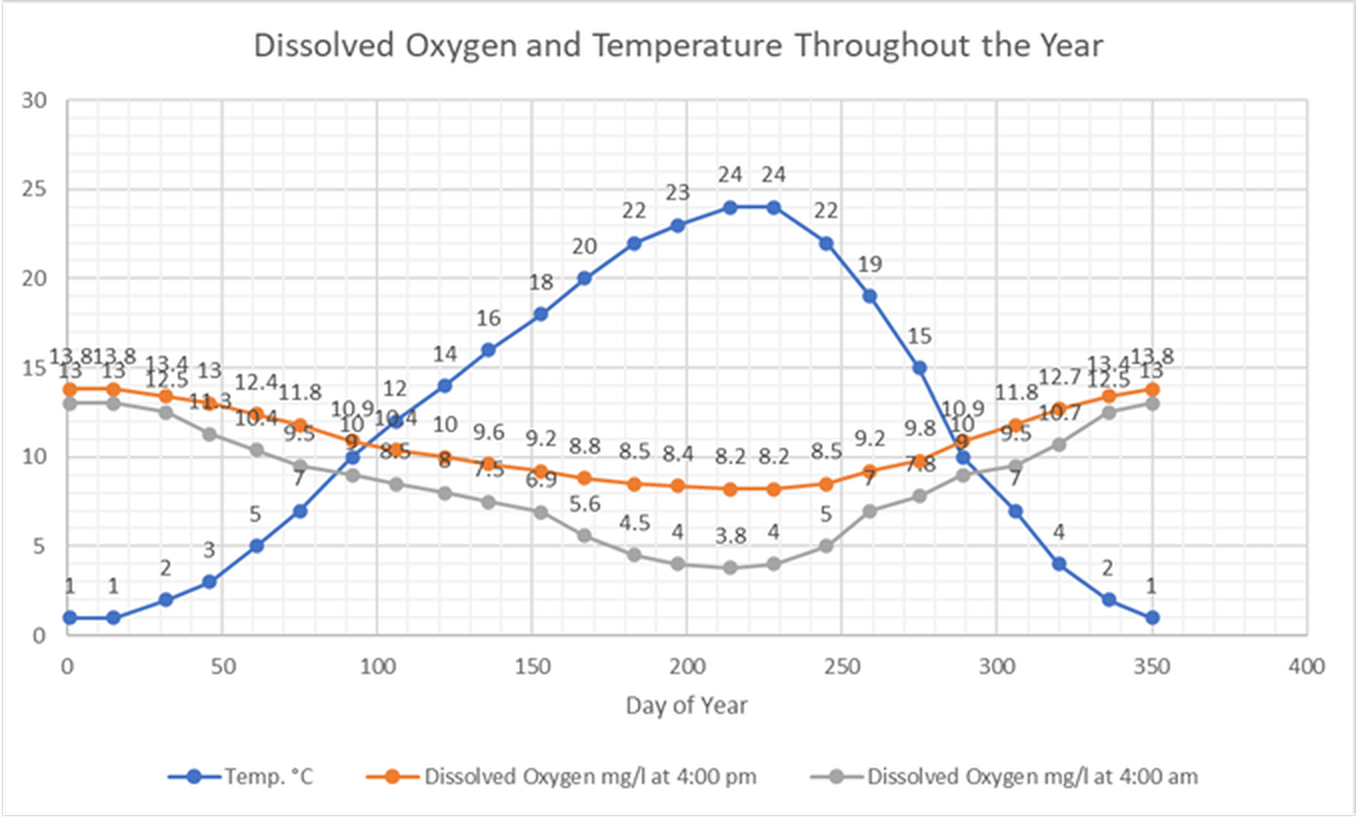
Changes in Temperature and Dissolved Oxygen Throughout a Year
The table below contains temperature and dissolved oxygen concentrations measured at the same site in a stream throughout an entire year.
The site has slow moving water, and aquatic plants grow in the soft sediments of the stream from spring through fall.
The first column of DO measurements were taken at 4:00 p.m. and the second column of DO measurements were taken at 4:00 a.m.
Have your students graph temperature and dissolved oxygen versus time.
Temperature is highest in summer, while DO is lowest in summer.
This is because saturation concentration of dissolved oxygen decreases as the water temperature increases.
Dissolved oxygen will be higher at 4:00 a.m. than at 4:00 p.m.
The plants in the water consume oxygen at night (due to metabolic respiration), but cannot produce oxygen from photosynthesis at night when there is not light.
Therefore, DO can be substantially lower in water at one time of day than another.
Dissolve Oxygen Concentrations Measured at the Same site in a Stream Throughout an Entire Year