What’s in the water?
Students observe and list abiotic factors in specific ecosystems. Students will observe and list abiotic factors in an aquatic system and measure four of them (pH, dissolved oxygen, turbidity, and temperature).
Purpose: To observe and list abiotic factors in specific ecosystems.
Background:
In this activity students will observe and list abiotic factors in an aquatic system and measure four of them (pH, dissolved oxygen, turbidity, and temperature).
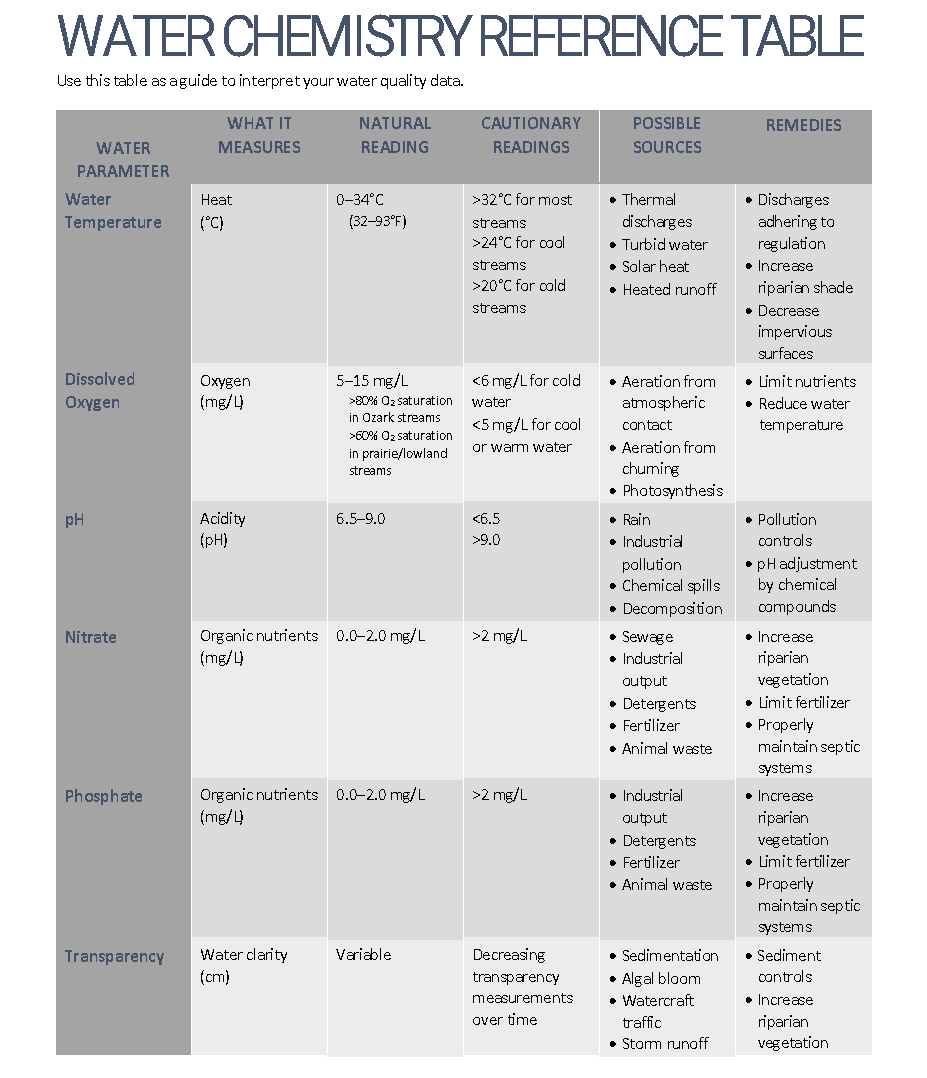
Physio-chemical indicators of water quality include dissolved oxygen, pH, temperature, dissolved solids, and nutrients (nitrogen and phosphorus).
Materials:
pH test kits
Dissolved oxygen test kits
Turbidity tubes
Thermometers
Copies of the student worksheet
Copies of the chemical sampling instruction sheets
Instruction Sheets
Water Quality Impact posters
Documents: Water chemistry
Presentations: Water chemistry

Activity:
- Set up a station for each factor (pH, DO, turbidity, and temperature).
At each station, provide:
• Sampling instruction sheets (if possible, laminate these!)
• The appropriate testing kit
• Samples in bottles if you are not near the stream - Divide the students into four groups. Explain to the students that each group will start at a different station, and rotate so they will measure all the factors.
- Have them follow the instructions for measuring each factor found on the sampling instruction sheets.
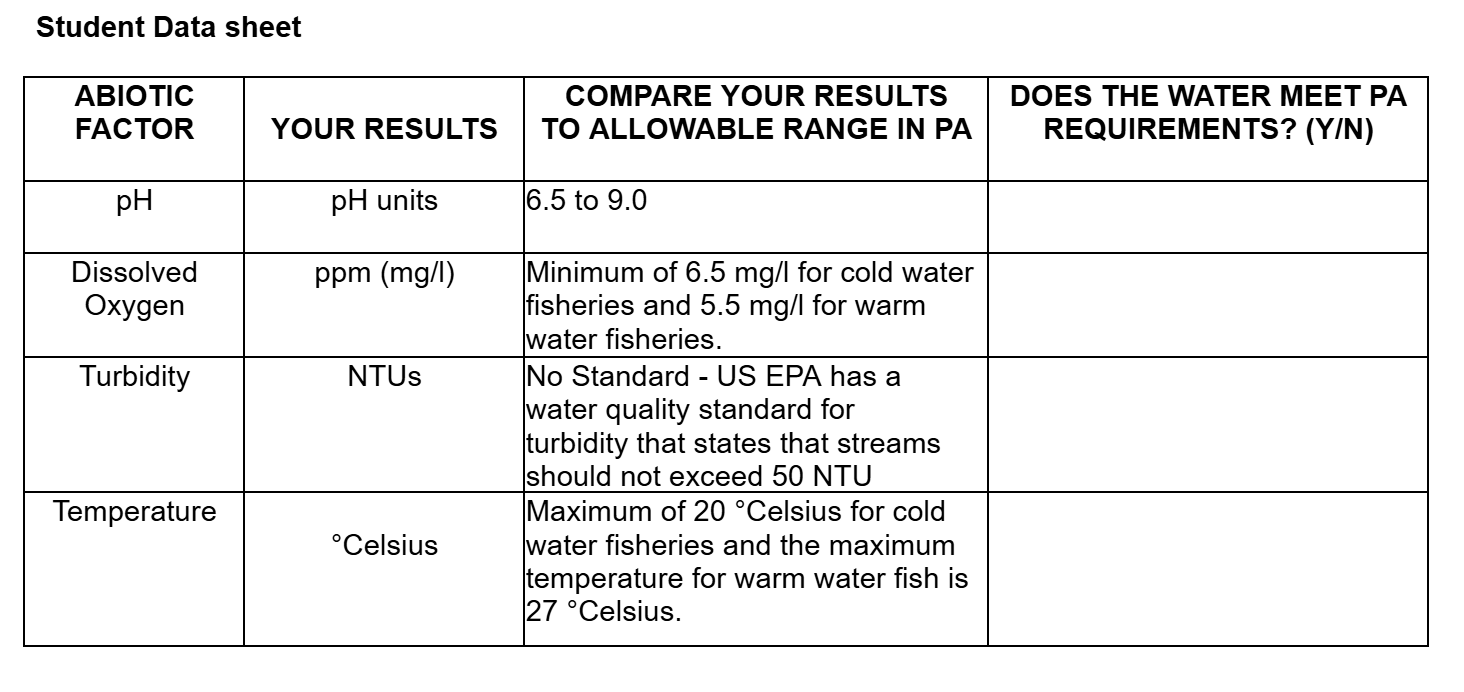
- Have the students record their results on the student worksheet.
ACTIVITY EXTENSIONS:
Use other water sources to compare results.
Discussion:
1. Does the stream meet water quality standards for:
- Dissolved oxygen?
- pH?
- Temperature?
- Turbidity?
2. How and why would previous weather conditions effect stream quality results?
3. What abiotic factors affect stream measurement results? How?
4. How might the following factors affect your stream analysis results?
5. Discuss the impact of the water quality parameters you measured on water quality of the stream
- Dissolved oxygen?
- pH?
- Temperature?
- Turbidity?
Create posters of the following to be used in the discussion.
pH of Water
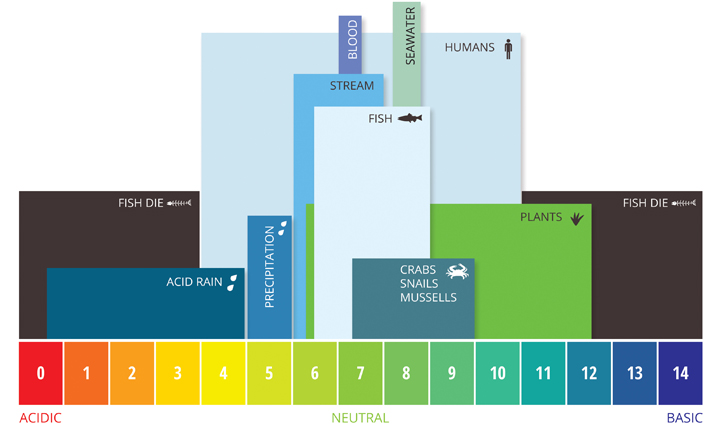
The majority of aquatic creatures prefer a pH range of 6.5-9.0.
If the pH of water is too high or too low, the aquatic organisms will die.
As pH levels move outside of the optimal pH range it can stress animal systems and reduce hatching and survival rates.
Low pH levels increase the solubility of elements and compounds, making toxic chemicals more “mobile” and increasing the risk of absorption by aquatic life
A slight change in a stream’s pH can increase the solubility of nutrients making them more accessible for plant growth.
pH levels over 9, the ammonium ion NH4 is converted to Ammonia NH3, which is extremely toxic to aquatic organisms.
Temperature
The stream temperature will vary throughout the year and through the day.
Stream temperature is influenced by the amount of water feeding into the stream from underground springs, by the amount of shade, by the volume of the water itself, and by the amount of agitation.
Stream temperature affects aquatic life’s sensitivity to toxic wastes, parasites, and disease, either through stress of rising water temperatures or the resulting decrease in dissolved oxygen.
Factors that affect stream temperature:
- Air temperature: The temperature of the air above the stream
- Sunlight: More sunlight warms the water
- Land use: Impervious surfaces like parking lots and roads heat up and run off into streams
- Water depth: Deeper water is cooler
- Water quality: Dirty water absorbs more heat from the sun
- Groundwater: The amount of groundwater that enters the stream
- Vegetation: Shade from trees along the stream bank cools the water.
Temperature ranges of some species:
Warm water species (70 degrees F and up) largemouth bass, crappie, bluegill, carp, catfish, caddisfly
Cool water species (65 to 70 degrees F) perch, sauger, walleye, smallmouth bass, pike, muskellunge, pickerel, rock bass, stonefly, mayfly, caddisfly, water beetles
Cold water species (70 degrees F and below) trout, salmon, caddisfly, stonefly, mayfly.
Impact of Turbidity on Aquatic Life
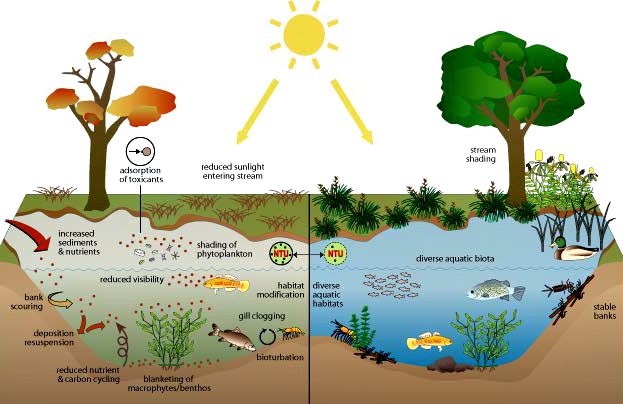
Reduced sunlight: Turbidity blocks sunlight from reaching plants, which reduces their ability to photosynthesize and produce oxygen. This reduces the amount of oxygen available to aquatic life.
Clogged gills: Turbidity can clog the gills of fish and other aquatic animals, making it difficult for them to breathe.
Reduced visibility: Turbidity can make it harder for predators to find prey.
Increased acidity: Decaying organic matter in turbid water produces carbonic acid, which increases the water’s acidity.
Reduced resistance to disease: Turbidity can reduce fish resistance to disease.
Altered egg and larval development: Turbidity can alter the development of fish eggs and larvae.
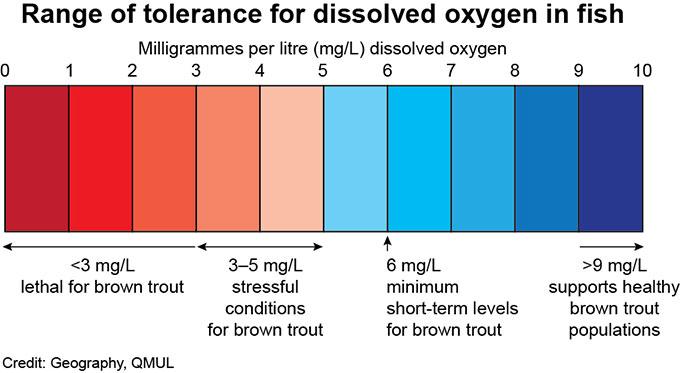
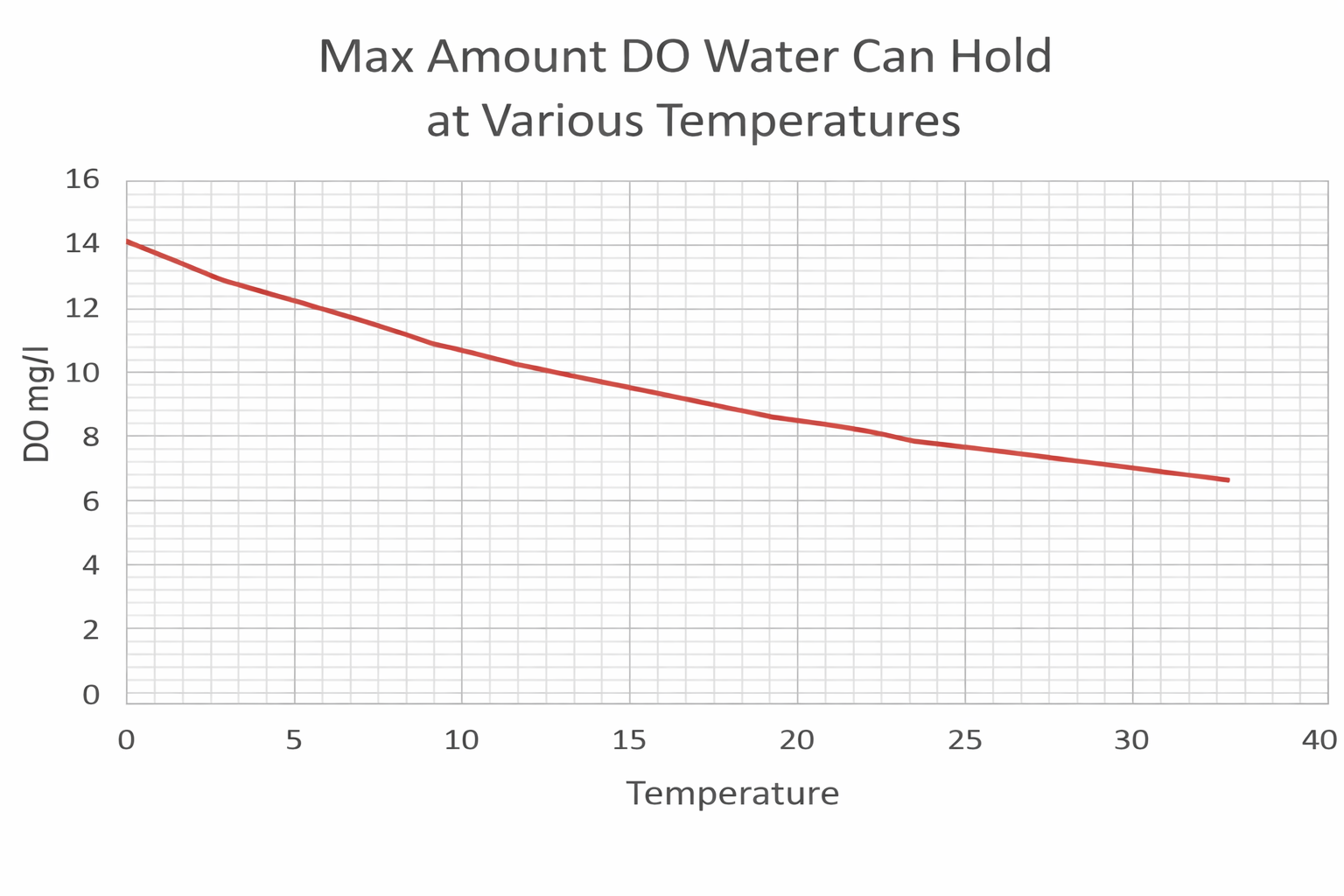
Healthy dissolved oxygen (DO) levels in streams are generally are above (6.5) to (8) mg/L and (80) – (120%) saturation, Coldwater fish, like trout need higher levels, typically at or above (6) mg/L, while levels below (3) mg/L can be dangerous and potentially lethal to aquatic life.
Why dissolved oxygen is important:
Supports aquatic life: Fish, insects, and other aquatic organisms require DO to survive and breathe.
Indicators of water quality: Low DO levels can signal poor water quality or pollution.
Habitat changes:
When DO levels drop, sensitive species like trout and mayfly nymphs may die or leave, replaced by organisms that tolerate low oxygen, such as sludge worms.
“Dead zones”: Extremely low DO levels create “dead zones” that cannot support most life.
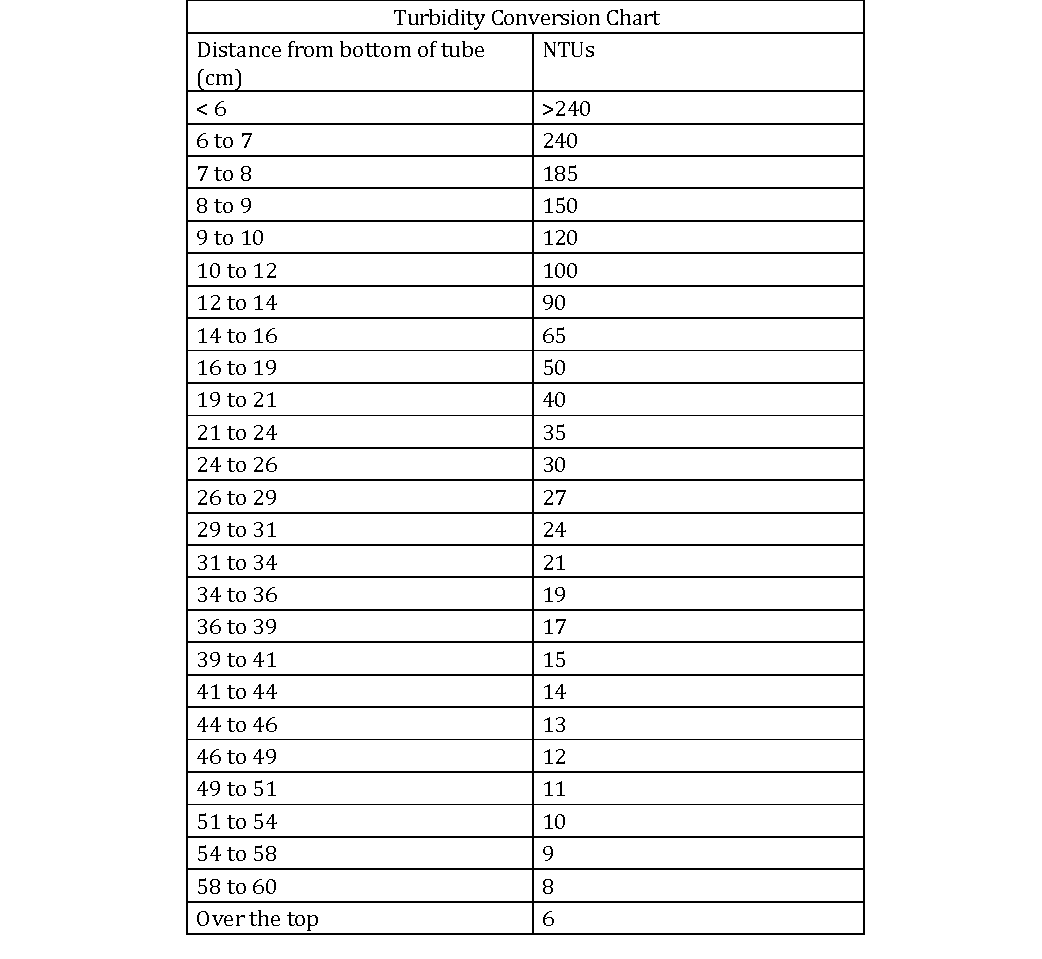
Turbidity Conversion Chart
Discussion:
1. Does the stream meet water quality standards for:
- Dissolved oxygen?
- pH?
- Temperature?
- Turbidity?
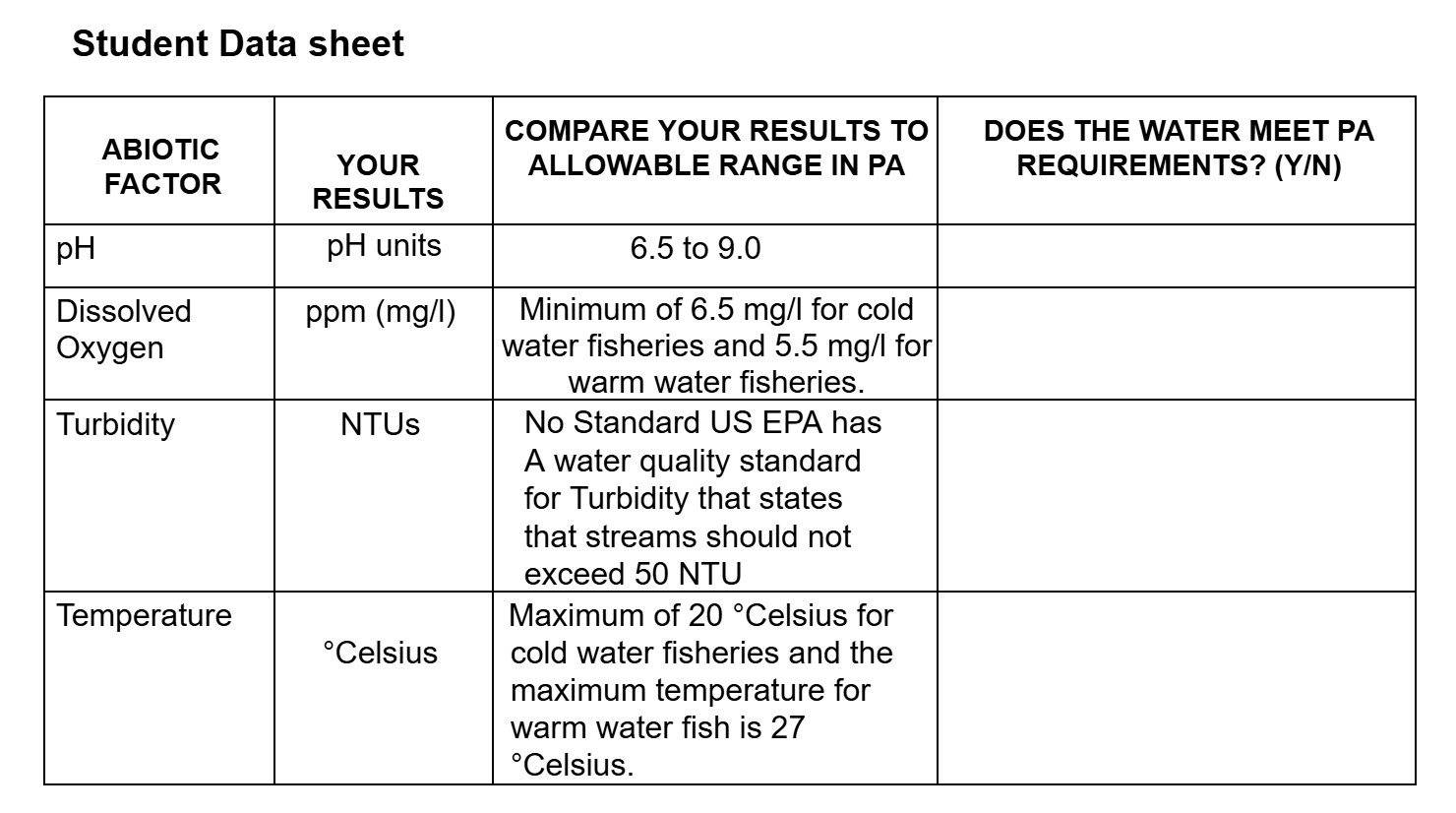
See the column of the student data sheet does the water meet PA requirements.
Considerer exploring with the students the reason(s) why the stream did not meet requirements.
2. How and why would previous weather conditions effect stream quality results?
Often it can take hours or days for the runoff from a storm or snowmelt to reach the water and travel down the river. Therefore, previous weather may be as important as today’s weather in explaining your results.
3. What abiotic factors affect stream measurement results? How?
Hot weather may result in extra snowmelt upstream and increase flows. Sunny weather may increase photosynthesis at your site, and therefore increase dissolved oxygen and pH levels. Higher flows from storms or snowmelt may increase the turbidity in your stream.
Soils in the watershed will affect the chemical composition of the runoff that reaches the stream. Topography (the steepness of the land) will determine whether the stream is steep and fast or slow and wide, which will affect dissolved oxygen and temperature.
Vegetation along the stream provides shade and protects the banks from erosion.
Land uses along the stream and in the watershed will determine what type of pollutants may enter the stream (e.g., sediment from agriculture or logging, metals and oils from roads, or fertilizers from golf courses).
4. How might the following factors affect your stream analysis results?
- Seasons
- From year to year
- Throughout the day
5. Discuss the impact of the water quality parameters you measured on water quality of the stream
- Dissolved oxygen?
- pH?
- Temperature?
- Turbidity?
